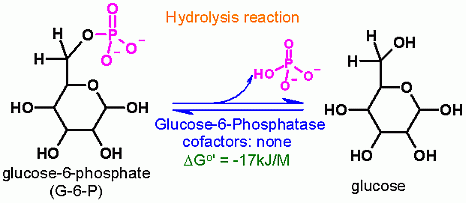
Glucose-1,6-bisphosphate synthase is a type of enzyme called a phosphotransferase and is involved in mammalian starch and sucrose metabolism (KEGG, 2.7.1.106 Archived 2011-07-16 at the Wayback Machine). It catalyzes the transfer of a phosphate group from 1,3-bisphosphoglycerate to glucose-1-phosphate, yielding 3-phosphoglycerate and glucose-1,6-bisphosphate.
(image courtesy of the BRENDA enzyme database)
The enzyme requires a divalent metal ion cofactor. Zinc (Zn2 ), Magnesium (Mg2 ), Manganese (Mn2 ), Calcium (Ca2 ), Nickel (Ni2 ), Copper (Cu2 ), Cadmium (Cd2 ) are all proven effective cofactors in vitro. Additionally, the enzyme appears to function optimally in a pH range from 7.3–8.7 and at a temperature of 25 °C.
Metabolic significance of the catalyzed reaction
The main product, glucose-1,6-bisphosphate, appears to have several functions:
1. Inhibition of hexokinase, an enzyme used in the first step of glycolysis.
2. Activation of phosphofructokinase-1 (PFK-1) and pyruvate kinase, both of which are enzymes involved in activation of the glycolytic pathway.
3. It acts as a coenzyme for phosphoglucomutase in glycolysis and gluconeogenesis.
4. It acts as a cofactor for phosphopentomutase, which produces D-ribose-5-phosphate. 5. acts as a phosphate donor molecule for unknown nonmetabolic effector proteins.
6. It increases in concentration during skeletal muscle contraction.
7. Its dephosphorylation yields glucose-6-phosphate, which is an important precursor molecule in glycolysis and the pentose phosphate pathway.
Glucose-1,6-bisphosphate is most likely used in correlation with gluconeolysis. The product’s inhibition of hexokinase and activation of PFK-1 and pyruvate kinase is indicative of its role in glycolysis. Glucose-1,6-bisphosphate inhibit hexokinase stopping the production glucose-6-phosphate from D-glucose. Its activation of PFK-1 and pyruvate kinase shows that glycolysis still continues without the production of glucose-6-phosphate from D-glucose. This means that the glucose-6-phosphate needed for glycolysis most likely comes from gluconeolysis.
The reactant glucose-1-phosphate is produced by gluconeolysis. This reactant can also form D-glucose-6-phosphate, which is needed for glycolysis. It can therefore be inferred that it is possible when glucose-1-phosphate is produced, it makes glucose-1,6-bisphosphate (with glucose-1,6-bisophosphate synthase) and glucose-6-phosphate. The glucose-1,6-bisphosphate increase the activity of glycolysis, of which glucose-6-phosphate is a reagent.
In addition, one of the reactants (1,3-bisphosphoglycerate) and one of the products (3-phosphoglycerate) are intermediates in the 'payoff' phase of glycolysis. In other words, two molecules involved with glucose-1,6-bisphosphate synthase are able to be both created and recycled in the glycolytic pathway.
The reactant glucose 1-phosphate is an important precursor molecule in many different pathways, including glycolysis, gluconeogenesis and the pentose phosphate pathway.
Regulation of the enzyme
Glucose-1,6-bisphosphate synthase is allosterically inhibited by inorganic phosphate, fructose-1,6-bisphosphate, 3-phosphoglycerate (a product), citrate, lithium, phosphoenolpyruvate (PEP), and acetyl CoA.
The inhibition of the enzyme by fructose-1,6-bisphosphate is most likely a feedback inhibition due to the product of the enzyme (glucose-1,6-bisphosphate) activation of PFK-1 (the enzyme which produces fructose-1,6-bisphosphate). When too much fructose-1,6-bisphosphate is produced, it inhibited the production of more PFK-1 activator.
The enzyme is also inhibited by PEP, which is a reagent of pyruvate kinase. The product of glucose-1,6-bisphosphate synthase (glucose-1,6-bisphosphate) activates pyruvate kinase.
Glucose-1,6-bisphosphate synthase appears to be activated by the presence of one of its substrates: 1,3-bisphosphoglycerate (glycerate-1,3-bisphosphate).
Enzyme structure
No structure determination of glucose-1,6-bisphosphate synthase has been documented to date. Nevertheless, studies have shown that its structure appears to be markedly similar to a related enzyme called phosphoglucomutase. Both enzymes contain serine linked phosphates in their active sites, both have the same molecular weights, and both require a metal ion cofactor. Perhaps most importantly, both enzymes produce glucose-1,6-bisphosphate as either a product or an intermediate.
Relevant links
KEGG: starch and sucrose metabolism with glucose-1,6-bisphosphate synthase (EC# 2.7.1.106)
http://www.genome.jp/dbget-bin/show_pathway?map00500 2.7.1.106
BRENDA enzyme database link for glucose-1,6-bisphosphate synthase (EC# 2.7.1.106)
http://www.brenda.uni-koeln.de/php/result_flat.php4?ecno=2.7.1.106 Archived 2011-07-16 at the Wayback Machine
Structure of phosphoglucomutase in the protein data bank
http://www.rcsb.org/pdb/explore.do?structureId=1LXT
References